Description
Delivered On: November 15, 2023
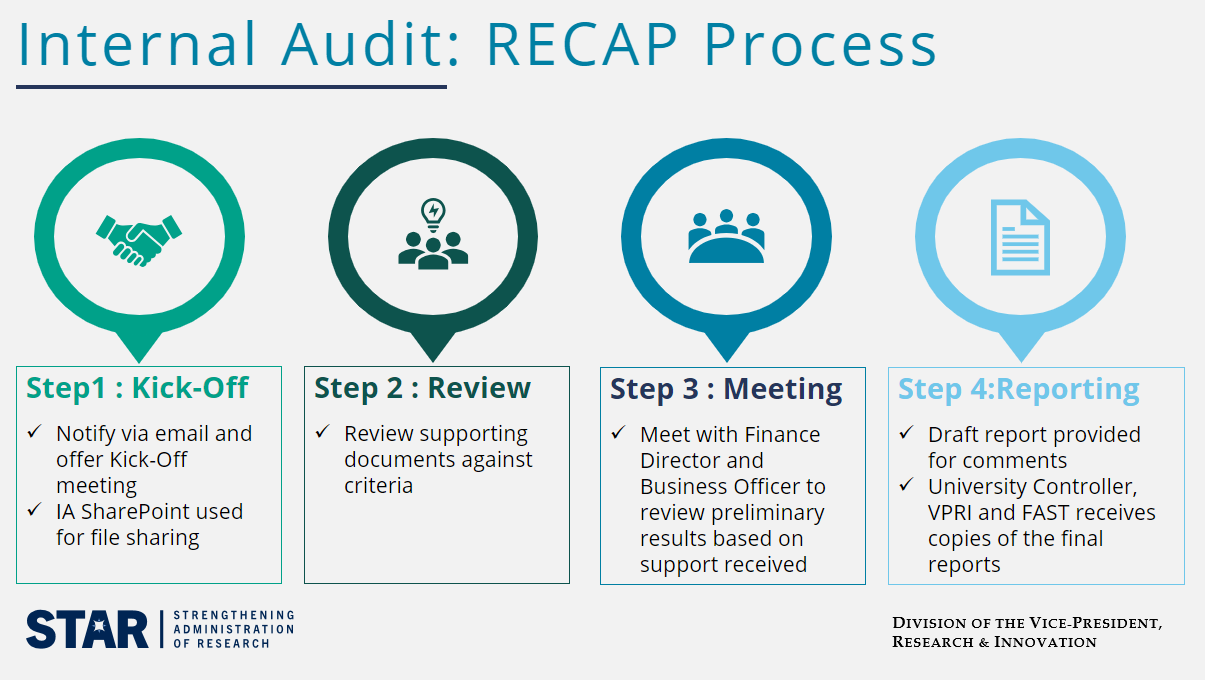
Join Internal Audit colleagues for an overview of their Research Expenditure Compliance Assessment Process (RECAP). Gain a better understanding of common internal controls and processes for improved compliance in research expenditures.
Learning Outcomes
- Understand key requirements for the appropriate use of grant funds.
- Recognize core elements of proper internal control procedures for improved compliance.
- Understand Internal Audit’s role and how they support research administrators
Key Takeaways
- It is important to maintain complete and clear documentation to substantiate the direct relevance of expenditures to the funded research activities; this includes ensuring that the grant-related purpose can be reasonably understood by an outside party.
- Research expenditure compliance is team effort. Grant-holders, counter-approvers and research administrators all play a crucial role in contributing to better internal controls by following established guidelines, policies, and procedures.
Presenters:
- Audelyn Budihardjo, Audit Manager, Internal Audit
- Lusine Amirkhanyan, Senior Auditor, Internal Audit
- Keziah Lo, Auditor, Internal Audit
Category:
Session Materials:


