BACKGROUND
The need for rapid, accessible, and reliable diagnostic tools for infectious disease is critical in both high-demand pandemic situations and for monitoring emerging threats (e.g. H5N1). Traditional diagnostic methods like qRT-PCR, while effective, often require specialized equipment and trained personnel, leading to delays in diagnosis. Novel synthetic biology point-of-care (PoC) diagnostics offer promising alternatives though they often rely on sample processing or signal detection devices that limit their translation. Microfluidic devices have the potential to reduce sample processing requirements by providing hands-free fluid manipulation in an integrated unit. Coupling microfluidic technology to a low-cost and accessible detection mechanism can bring such PoC devices to new contexts, such as pharmacies, clinics, border crossings and the clinic.
TECHNOLOGY
Researchers at the University of Toronto have developed an automated sample-to-answer microfluidic platform that integrates a cell-free glucogenic gene circuit assay for molecular diagnostics (Figure 1). The microfluidic platform automates the entire diagnostic process, from sample input to result readout. It uses microfluidic technology to transfer, meter, and mix assay components, performing the test with minimal manual intervention. The system includes a sample metering tool, RNA extraction using a lysis buffer, amplification via reverse transcription loop-mediated isothermal amplification (RT-LAMP) via a simple onboard heater, and detection using a cell-free system (CFS) with a toehold switch that generates a glucose signal. The glucose output is measured using a standard glucose meter, making the assay accessible and easy to use for untrained or minimally trained individuals.
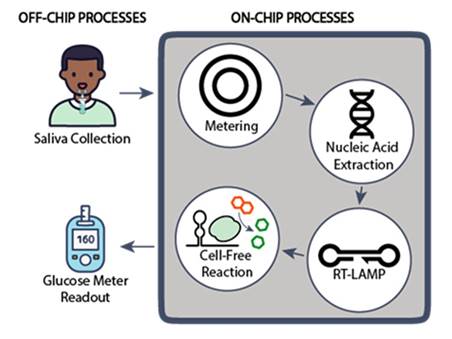
Figure 1. Pathogen detection using an automated microfluidic system with glucose meter readout. A collected saliva sample is metered, to standardize the input volume, before the RNA is extracted by mixing it with a lysis buffer. The RNA is then amplified using RT-LAMP. A cell-free (CF) protein expression reaction employing a toehold switch then translates the specific nucleic acid target into a glucogenic reporter signal, which can be read by a standard glucose meter. All sample metering, mixing, heating and transfer steps happen on-board the device.
COMPETITIVE ADVANTAGE
- Automated Process: Reduces the need for technical training and specialized equipment.
- Low-Cost Output: Utilizes a standard glucose meter for readout, eliminating the need for expensive lab equipment.
- High Sensitivity and Specificity: Comparable to gold-standard qRT-PCR tests.
- Portable and User-Friendly: Designed for use in remote and low-resource settings.
- Multiplexing Capability: Potential for detecting multiple targets in a single assay.
- Mass Manufacturable: Device chip is produced by standard injection molding methods, single-layer structure, on-board heating, with low energy consumption.
APPLICATIONS
- Disease Detection: Rapid and accessible testing for various pathogens in community-based settings.
- Low-Resource Environments: Diagnostic tool for areas lacking advanced medical infrastructure.
- Point-of-Care Testing: Convenient and efficient testing at the site of patient care.
- Environmental Monitoring: Detection of pathogens or contaminants in environmental samples.
INTELLECTUAL PROPERTY STATUS
- Provisional patent application (March 2025)
PROJECT STATUS
The microfluidic platform has been successfully developed and tested with SARS-CoV-2 patient saliva samples, demonstrating sensitivity and specificity comparable to qRT-PCR.
KEYWORDS
Automated Microfluidic System, Glucose Output, Molecular Diagnostics, Point-of-Care Diagnostics, Synthetic Biology, RT-LAMP, Cell-Free System, Toehold Switch, Low-Cost Diagnostic, Disease Detection, Remote Testing, Multiplexing Capability