BACKGROUND
As of today, research utilizing genuine SARS-CoV-2 virus must be conducted in biosafety level 3 (BSL3) labs, to which few facilities have access. As a result of biosafety concerns, researchers rely on highly reductive minimalistic tools that enable studying limited aspects of viral life cycles in isolation. Such tools include the use of spike protein-pseudotyped viral particles to study viral entry and various reporter assays for SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) or proteases. While such tools are useful and biosafe to use in BSL1/2 labs, they suffer from multiple drawbacks. These assays reproduce a single step in the viral life cycle in isolation from the multitude of events that occur simultaneously in a genuine virus infection. Conversely, genuine SARS-CoV-2 assays are far from being well suited for high throughput screening of large chemical libraries. Additionally, many of genuine SARS-CoV-2 infection and replication assays are traditionally conducted in non-human cell lines due to poor performance in human cell lines. While these non-human cell lines are important, their use carries the risk of obtaining results that are irrelevant to human due to different genetic and proteomic makeup.
TECHNOLOGY
Researchers at the University of Toronto have developed a SARS-CoV-2 replicon that achieves three goals:
- Ensured biological safety by mutating genes that are known to be essential for the ability of SARS-CoV-2 to generate infectious viral particles
- Engineered the replicon in a manner that renders it highly adaptable for high throughput screening facilities
- The design ensures full functionality of SARS-CoV-2 molecular replication, polypeptide maturation and organization thus rendering it a high fidelity biologically safe and highly adept tool for screening for inhibitors of SARS-CoV-2.
COMPETITIVE ADVANTAGE
- The replicon is integrated in human cell lines and requires no production or in vitro transcription.
- Highly suited for HTS
- Replicon is built with several reporters that suite a wide variety of detection instruments (luminescence and fluorescence at different wavelengths)
- Replicon is approved by PHAC (Public Health Agency of Canada) for use in CL2 labs
- Trans-complementation can be performed in a manner that provides an improved tool to study the natural progression of SARS-CoV-2 as genuinely as possible. In other published replicons that can be complemented, the manner in which this is possible is irrelevant to the natural replication process of SARS-CoV-2, which limits the utility of complementation in those systems
- Only replicon available to date that is integrated in human cell lines and is approved for use in CL2 labs
APPLICATIONS
- High throughput screening for inhibitors of viral replication. This encompasses all of the biochemical processes required to achieve this, including:
- Viral proteases-mediated proteolysis
- RdRp activity
- Nested transcription and translation
- High throughput screening for inhibitors of cellular processes essential for SARS-CoV-2 replication. Theoretically, dysregulation of these processes may provide a window where viral replication is inhibited while compensatory pathways salvage cellular homeostasis.
- Trans-complemented SARS-CoV-2-like virus particle applications: These virus-like particles are nearly morphologically and proteomically identical to genuine SARS-CoV-2 which can be used in:
- Studies of inhibitors of viral entry.
- Potential use as vaccines, which has the whole host of viral proteome and most of its genome (albeit replication-defective). This is expected to illicit a varied and multi-pronged immune response closely related to a genuine infection without the health hazards due to defective virus replication.
INTELLECTUAL PROPERTY STATUS
- Proprietary Cell Line
PROJECT STATUS
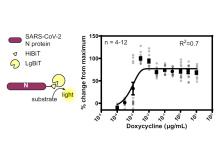
Replicon has been integrated in human cell lines. Replicon induction has been tested and shown to provide a robust HTS-compatible assay (Z’>0.6). Replicon cells have been successfully induced and used for testing novel and classic compounds that were shown effective against SARS-CoV-2. Replicon safety has been demonstrated experimentally. Subsequently, replicon-integrated cells have been approved for use in BSL2 laboratory by Public Health Agency of Canada.