BACKGROUND
The co-delivery of colloidal small molecule and ribonucleic acid (RNA) drugs is an innovative approach in therapeutic development. This strategy combines the benefits of small molecule drugs, which can inhibit specific proteins, with RNA therapeutics like small interfering RNA (siRNA), microRNA (miRNA) or mRNA. Studies have shown that co-delivery systems can overcome drug resistance and improve therapeutic outcomes by synergistically targeting multiple pathways involved in disease progression. Recent advancements in nanocarrier technology have enabled the simultaneous delivery of these drugs, enhancing their stability and targeting efficiency. However, conventional liposomal or polymeric delivery systems used for small molecules are inefficient for RNA delivery while lipid nanoparticles (LNPs) typically used for RNA delivery are unfavourable for small molecule drug loading (usually less than 10 wt% drug).
TECHNOLOGY
Researchers at the University of Toronto have developed high-content drug-RNA nanoparticles by replacing the ionizable lipid of conventional LNP platforms for nucleic acid delivery with an ionizable, colloid-forming drug, thereby resulting in novel and high-content drug-RNA nanoparticles. The ionizable drug facilitates RNA encapsulation in the nanocarrier via electrostatic complexation, while also facilitating endosomal disruption and drug/RNA release after cellular uptake. Using ionizable analogs of the commercially approved colloidal drug fulvestrant, they have demonstrated codelivery of drug and siRNA (targeting the undruggable CCNE1) in breast cancer cells exhibiting a reduced response to fulvestrant. This technique is limited to colloidal small molecule drugs that possess a chemical moiety sufficient distant from the molecules’ active site that can be chemically modified, for ionizable group introduction, and still retain potency.
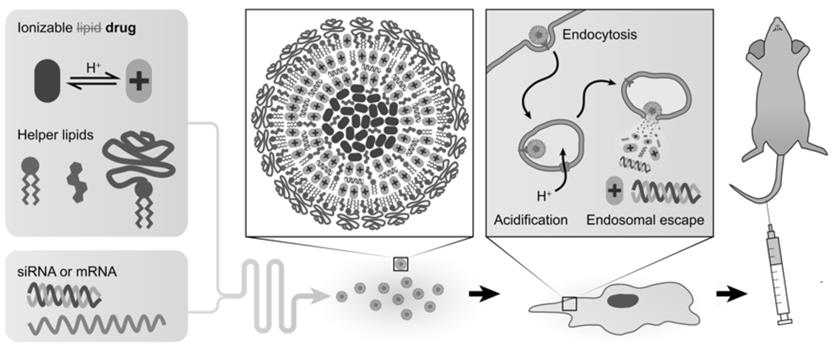
Figure 1. Ionizable drug-RNA nanocarriers for synergistic therapeutic targeting. The ionizable drug replaces the ionizable lipid in LNPs, while all other components remain the same. The formed nanoparticles possess a colloidal drug-heavy core that helps encapsulate the RNA. Cellular uptake in endosomes results in a change in pH that triggers protonation of the ionizable drug and disruption of the membrane, triggering endosomal release.
COMPETITIVE ADVANTAGE
- Synergistic targeting of multiple pathways
- High encapsulation efficiency (~80% of siRNA)
- High loading of ionizable drug (>50%)
- Several other favourable delivery system properties:
- Comparable cellular uptake to MC3-LNPs (SKOV3 cells)
- Cytosolic delivery of siRNA
- IC50 ~ 10 nm (luciferase (FLuc) knockdown, SKOV3-FLuc cells)
- Stable in storage (~ 2 years at 4°C in terms of diameter)
- Broad applicability to other ionizable small molecule drugs
APPLICATIONS
- Combination Therapeutics
- Colloidal small molecule drugs + RNA therapeutics
INTELLECTUAL PROPERTY STATUS
- US and CA Applications (July 2024)
PROJECT STATUS
Proof-of-concept studies have been conducted in vitro and in vivo to demonstrate the utility of ionizable drugs for RNA therapeutic delivery. A Fulvestrant analog was used as a model ionizable drug that enabled encapsulation of siRNA in drug-RNA rich nanoparticles. The combination therapy has been tested on a fulvestrant-resistant breast cancer cell line.