Uncemented orthopedic implants frequently experience aseptic loosening, primarily due to insufficient bonding with biological tissues. In response to this limitation, the research team has developed an innovative, personalized solution for uncemented joint replacement featuring a calcium polyphosphate coating that can be incorporated into 3D-printed titanium alloy implants. This approach leverages advanced imaging modalities such as computed tomography (CT) or magnetic resonance imaging (MRI) to design a customized joint replacement model tailored to the patient's anatomy.
The implant is fabricated through additive manufacturing techniques and further optimized for osseointegration using a proprietary electrodeposition method for calcium polyphosphate coating. This novel solution is anticipated to offer enhanced biocompatibility compared to standard off-the-shelf implants, while also reducing production time and costs relative to existing personalized implant options.
BENEFITS
The novel solution is engineered to have lower loosening and wear risk, ultimately with lower revision risk. The key feature is using the electrodeposition technique to produce calcium polyphosphate coating onto a 3D-printed titanium alloy implant. The team is the first group using electrodeposition to conduct calcium polyphosphate coating on biomedical devices (see Figure 1: Electrodeposition of Calcium Polyphosphate (preliminary results)).

This method combines two bioactive materials for faster osseointegration, higher biocompatibility, higher precision, and lower manufacturing cost. The as-deposited honeycomb porous surface has an average pore size of around 100μm, allows tissues and bone cells grow into the coating for long-term biological bonding. The synthesized samples have been characterized through SEM, EDS, XRD, and tested by micro- and nano-hardness indentation. The osseointegration property was verified through a 4-week body-simulated fluid soaking, a layer of calcium deposition was confirmed on the surface.
Meanwhile, the additive manufacturing technique enables a faster fabrication process compared with subtractive manufactured personalized implants, shortening the wait time from 6 weeks to 2 weeks.
Furthermore, the optimized interior structure can be tailored to fit the human bone mechanical properties to avoid the stress-shielding effect and bone loss (see Figure 2: Personalized Implant Stem Manufacturing Process).
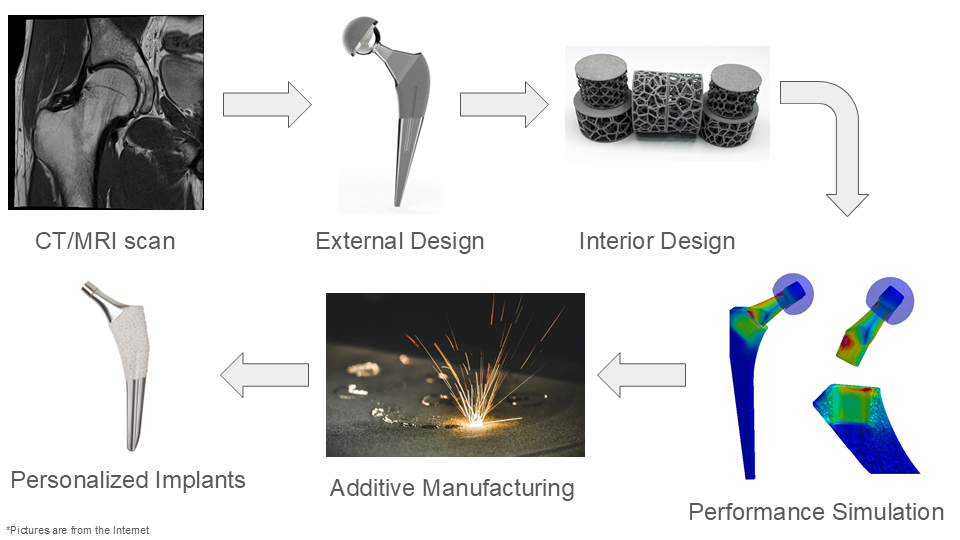
APPLICATIONS
More than 58,600 hip replacements are performed in Canada each year, and 42% of hip replacements due to fracture were fixed by cementless method. If the original implants fail after years, a revision surgery will be required. revision surgery requires the removal of old implants and replacing them with a new one. It is normally more complex, expensive, and requires a longer inpatient stay than the primary surgery. Considering the patients’ median age for joint replacement surgery is 65, a revision replacement would significantly increase the surgical risk and postsurgical recovery difficulty.
In 2023, the joint replacement market was estimated at $22.3B USD, growing to over $38B by 2030 with a CAGR of 7.9%. North America, as one of the largest joint replacement markets, took a 36% share of the global market at $7.5B. In this TAM, personalized joint replacement is an emerging market with a value of $1.9B in 2022, expected to reach $2.7B by 2029.
OPPORTUNITY
IP has been filed to support successful grant applications and the team has successfully synthesized uniform, crack-free coated samples and has preliminarily evaluated their osseointegration capabilities using body-simulated fluid assays. Now seeking strategic partners for further validation, licensing, and startup support.
Image credits:
[1] Radiopaedia, “| Playlist ‘UQ Med Yr 1 GAF/Radiographic Anatomy - Lower limb’ by Assoc Prof Craig Hacking,” Radiopaedia, 2025. https://radiopaedia.org/play/16096/entry/261473/case/42600/studies/45743 (accessed Jan. 10, 2025).
[2] S. McConnell, “to portray. Prosthetic Hip Implant,” grabcad.com, Feb. 08, 2016. https://grabcad.com/library/prosthetic-hip-implant-1 (accessed Jan. 10, 2025).
[3] Lubrizol, “Design for Additive Manufacturing with Lattice Structures - Lubrizol,” www.lubrizol.com. https://www.lubrizol.com/3D-Printing/Blog/2021/08/Design-Lattice-Structures-with-AM (accessed Jan. 10, 2025).
[4] E. Group, “ESI Software Solutions Benefit the Biomedical Sector,” ESI Group, Apr. 02, 2015. https://www.esi-group.com/news/esi-software-solutions-benefit-the-biomedical-sector (accessed Jan. 11, 2025).
[5] “Feeling the heat — Kanthal®,” Kanthal.com, 2020. https://www.kanthal.com/fr/centre-de-connaissances/t%C3%A9moignages-inspirants/feeling-the-heat/ (accessed Jan. 10, 2025).
[6] Stryker, “Accolade II,” www.stryker.com, Mar. 2024. https://www.stryker.com/us/en/joint-replacement/products/accolade-ii.html (accessed Jan. 10, 2025).





