BACKGROUND
Injuries to the central nervous system, such as stroke and traumatic spinal cord injury, result in an aggregate scar that both limits tissue degeneration and inhibits tissue regeneration. The aggregate scar includes chondroitin sulphate proteoglycans (CSPGs), which impede cell migration and axonal outgrowth. Chondroitinase ABC (ChASE) degrades CSPGs and could therefore be used to facilitate tissue regeneration, but the native enzyme is fragile.
TECHNOLOGY OVERVIEW
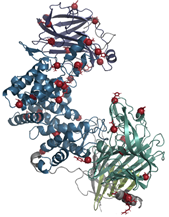
A mutant of ChASE was designed with 37-point mutations to the native enzyme (ChASE37), that displays improved stability and activity, to degrade inhibitory chondroitin sulfate proteoglycans (CSPGs) (Figure 1). The enzyme’s efficacy was tested in vitro using human cell-based assays and in vivo in a rodent model of stroke, showing promising results for tissue regeneration. An injectable hydrogel system was also created for the prolonged release of ChASE37, enabling its bioactivity in the stroke-injured rat brain. This technology lays the groundwork for future research into the potential of ChASE37 and its delivery system for tissue regeneration after stroke and other CNS injuries.
Figure 1. Computational modeling was used to suggest 37 amino acid mutations (red balls) in chondroitinase ABC producing the ChASE37 mutant.
COMPETITIVE ADVANTAGE
- Enhanced ChASE enzyme: ChABC-37 mutant exhibits properties that could promote its therapeutic uses including, a ~ 6-fold increase in stability and ~50% higher enzymatic activity compared to wild type.
- CSPG Degradation: ChASE37 effectively breaks down CSPGs, facilitating cell adhesion and neuronal growth (~ 5-fold greater area of digested CSPGs in stroke-injured rat brain).
- Hydrogel Delivery: A custom hydrogel ensures controlled release of SH3-ChASE37, maintaining its activity in vivo.
- Neurite Outgrowth: Treatment with SH3-ChASE37 enhances neurite number and length, promoting neuronal differentiation.
- In Vivo Bioactivity: SH3-ChASE37 maintains bioactivity in a stroke-injured rat brain, suggesting tissue regeneration potential.
- Immune Response: SH3-ChASE37 does not trigger an immune response, indicating its potential safety for therapeutic use.
APPLICATIONS
- Treatment for traumatic injury to the central nervous system
- Stoke and spinal cord injury
INTELLECTUAL PROPERTY STATUS
- US (17/798,415) and Canadian (3,167,489) national applications pending.
PROJECT STATUS
Disease animal studies have been conducted. Our findings suggest that the affinity-released SH3-ChASE37 from hydrogels is bioactive and can degrade CSPGs in the stroke-injured rat brain without eliciting an immune reaction.
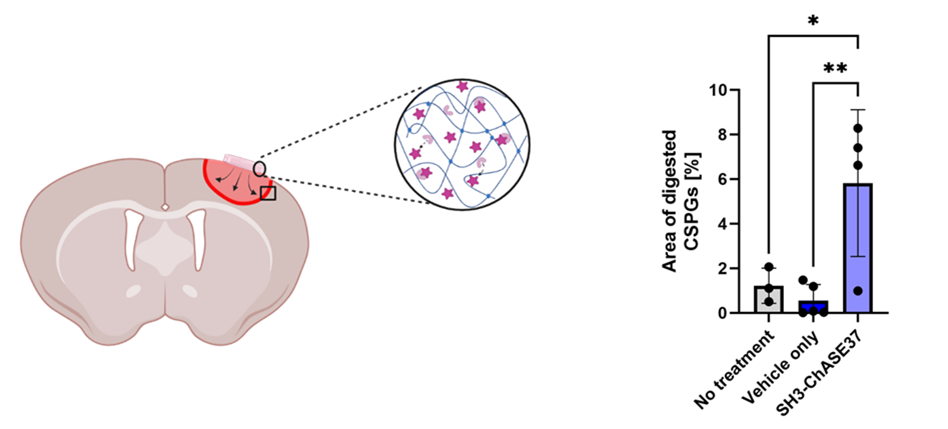
Figure 2. Affinity-released SH3-ChASE37 degrades CSPGs in the stroke-injured rat brain. Schematic of the delivery of SH3-ChASE37 from CMC-bp (carboxymethylcellulose hydrogel with SH3-binding peptides) injected in the epicortical space (left). Quantification of the positive area of digested CSPGs at day 7 (right).