BACKGROUND
Fungal infections commonly occur and can be associated with significant morbidity and mortality in patients that are immunocompromised such as those undergoing chemotherapy, organ transplant recipients, and people with HIV/AIDS. Four classes of antifungal agents exist for the systemic treatment of mycoses, however increasing rates of resistance, host toxicity and a limited spectrum of activity has created a need for new treatments, and especially for those with new mechanisms of action. The essential role of the molecular chaperone Hsp90 in the three species (Candida albicans, Aspergillus fumigatus, and Cryptococcus neoformans) responsible for >90% of invasive mycosis may make it an attractive target, if selective molecules can be designed that evade the human Hsp90 paralogs.
TECHNOLOGY
Researchers at the University of Toronto have developed the first inhibitors selective against fungal Hsp90. The starting point to design was a lead compound derived from resorcylate macrocycle natural products. A structure-activity relationship (SAR) program was conducted to guide the development of a resorcylate aminopyrazole (RAP) amide scaffold (Figure 1) with high efficacy and selectivity towards C. neoformans and C. albicans Hsp90. Structure-property optimization was then used to develop molecules for additional drug-like properties such as cell permeability.
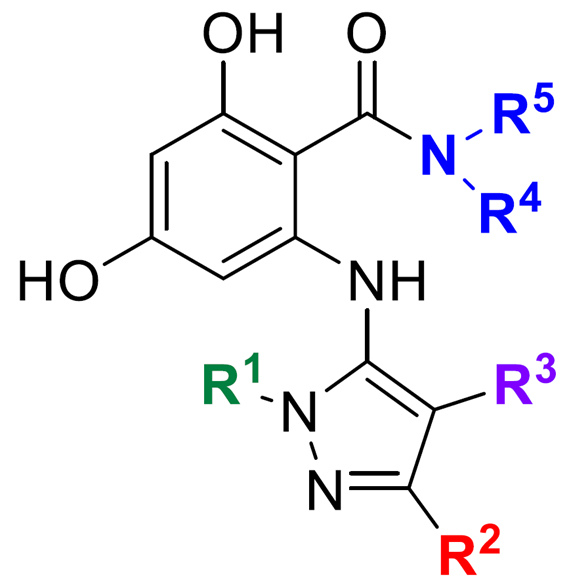
Figure 1. Structure of the resorcylate aminopyrazoles (RAPs) amides with fungal-selective Hsp90 inhibitory activity.
COMPETITIVE ADVANTAGE
- First class of selective Hsp90 inhibitors
- Capable of discriminating between fungal and human isoforms of Hsp90
- Broad, potent, and fungal-selective Hsp90 inhibitory activity
- C. neoformans
- Selectivity ~ 5 – 30 fold
- EC50 ~ low – mid nM (cell lysate)
- MIC80 ~ low µM (whole cell)
- Intermediate Microsomal Stability
- T1/2 ~ 20 – 30 min
- CLint ~ 20 – 70 µl/min/mg
- C. albicans
- Selectivity ~ 5 – 20 fold
- EC50 ~ mid nM (cell lysate)
- MIC80 > 50 µM (whole cell; permeability not optimized)
- C. neoformans
APPLICATIONS
- Antifungal agents
INTELLECTUAL PROPERTY STATUS
- Patent Documents: CA, US (granted patent)
PROJECT STATUS
This program is at the lead-optimization stage. Structure-activity (i.e. potency and selectivity) and structure-property (e.g. cell permeability) rounds of optimization have been conducted. Further development is ongoing to generate molecules with greater drug-like properties.