BACKGROUND
Genome editing with nucleases such as Cas9 relies on homology-dependent repair (HDR) for the targeted insertion or replacement of genetic material. However, as HDR competes with other double-strand break (DSB) repair pathways such as non-homologous end joining (NHEJ), HDR efficiency can often be limiting, posing a challenge to achieving desired levels of genomic alterations. One way to overcome this deficiency would be to inhibit NHEJ during genome editing. A prime target may be 53BP1, a key regulator of DSB repair pathway choice in eukaryotic cells. It functions to favor NHEJ over HDR by suppressing end resection, which is the rate-limiting step in the initiation of HDR. Inhibitors of 53BP1 may thus increase HDR efficiency during genome editing.
TECHNOLOGY
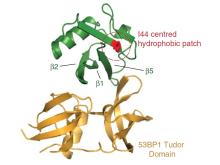
Our researchers have developed a genetically encoded inhibitor of 53BP1 that increases the efficiency of homology-directed repair of DSBs. As 53BP1 accumulates at DSB sites by recognizing a ubiquitylated histone, the researchers postulated that a ubiquitin variant might act as a possible inhibitor. To develop the inhibitor they used a combinatorial library to generate engineered ubiquitin variants and identified one variant, i53 (inhibitor of 53BP1), that reduced accumulation 53BP1 at sites of DNA damage and increased gene conversion with either double-stranded DNA or single-stranded oligonucleotide donors by up to 5.6-fold.
COMPETITIVE ADVANTAGE
- Strong Inhibitor (~ high nM)
- Strongly suppresses recruitment of 53BP1 to DSB sites
- Two orders of magnitude tighter binding than the 53BP1 – histone (H4K20me2) interaction
- Contact surfaces are extensive
- Buried surface area = 755.4 Å2
- Comprised a mixture of hydrophobic and hydrophilic residues
- Increases gene conversion efficiency
- Cas9 (AAV)
- dsDNA
- 1.3-fold to 2.5-fold increase in gene conversion (dependent on cell line and locus)
- 1.9-fold increase in bi-allelic gene conversion
- ssODN
- upto 3.3-fold increase (dependent on cell line and locus)
- dsDNA
- ZNF (electroporation)
- Upto 5.6 fold (dependent on cell line and locus)
- Cas9 (AAV)
- Only binds to 53BP1(immunoprecipitation–mass spectrometry experiments)
- Independent of nuclease (Cas9, ZFN), delivery methods, or single-stranded/double stranded oligodeoxynucleotide donors
- Better than tested competition
- Compared to both SCR7 and to SCR7 pyrazine
- Likely effective in many organisms (tested in human and murine cell lines)
APPLICATIONS
- Gene Therapy
INTELLECTUAL PROPERTY STATUS
- National Phase (2018): US (Granted), EU, CA
PROJECT STATUS
Proof-of-concept studies have been conducted. An inhibitor of 53BP1 (i53) was isolated from a combinatorial screen and characterized using biochemical and biophysical techniques. i53’s potential to increase gene conversion has been tested in multiple cell lines (both human and murine) and at different loci.