BACKGROUND
Diabetes, a group of metabolic diseases marked by hyperglycemia, affects 422 million people globally. Insulin-dependent patients need multiple daily injections of insulin to manage their blood sugar. However, over administration of insulin doses can cause acute hypoglycemia, a life-threatening complication that requires emergency injections of glucagon. Such injections require significant patient intervention, which is particularly challenging for those with hypoglycemia-unawareness, nocturnal hypoglycemia, or those dependent on caregivers. Therefore, there’s a pressing need for automated glucagon delivery systems to reduce the frequency and risk of hypoglycemia.
TECHNOLOGY OVERVIEW
Researchers at the University of Toronto have developed a composite microneedle patch capable of releasing glucagon in response to hypoglycemia (Figure 1). Each microneedle is loaded with multifunctional microgels that enable efficient glucagon loading and stabilization, and hypoglycemia-triggered release. When the microneedle patch is applied, the microneedles penetrate the skin allowing the microparticles to interact with glucose. In the presence of normal levels of glucose, the microparticles remain swollen resulting in limited release of glucagon. However, under hypoglycemic conditions, the reduction of glucose levels triggers microparticle contraction releasing glucagon in sufficient quantities to stabilize glucose concentrations. The entire process proceeds automatically without external intervention of patients or caregivers.
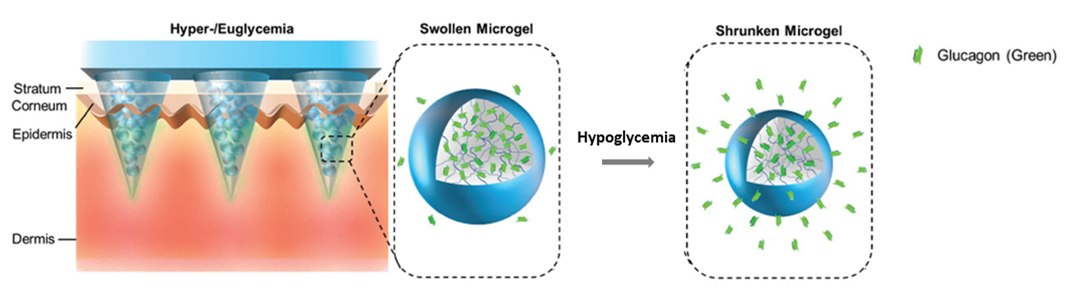
Figure 1. A microneedle patch loaded with glucagon-containing microgels. Reduction in glucose levels triggers a contraction of the microgel and release of glucagon.
COMPETITIVE ADVANTAGE
- Automatic delivery of glucagon
- No external intervention required, either by patient or caregivers.
- Prevents hypoglycemia
- Maintains blood-glucose-levels at safe levels (~100 mg/ml) for at least 2.5 hrs in rats
- Microgel maintains structure and activity of native glucagon
- Easy application and removal
APPLICATIONS
- Diabetes
- Hypoglycemia
- Hypoglycemia
INTELLECTUAL PROPERTY STATUS
- National Phase: CA, EP, US
PROJECT STATUS
Proof-of-concept studies have been conducted in T1D rat models (Figure 2).
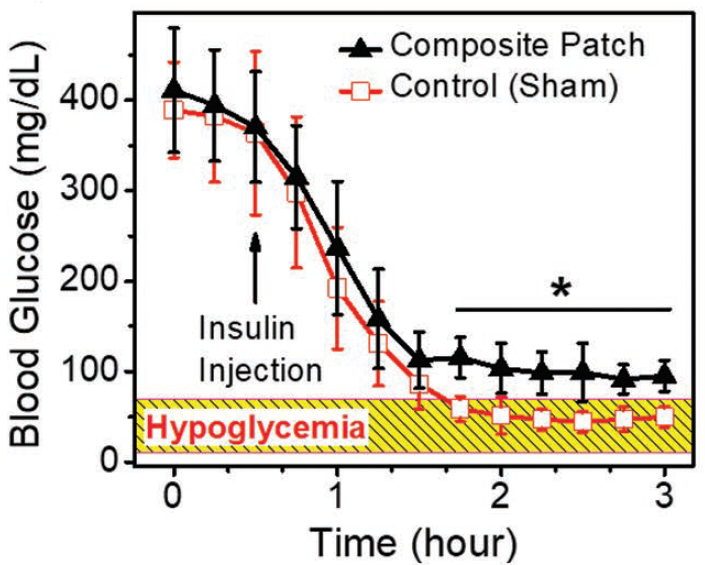
Figure 2. Comparison of a composite microneedle (cMN) patch and a sham device in T1D rats treated with a high dose of insulin to induce hypoglycemia. Use of the cMN patch maintains glucose levels at ~ 100 gm/dL, above hypoglycemic conditions.