BACKGROUND
Deep skin wounds, such as those caused by burns, are usually treated by harvesting skin from healthy regions of the body and redistributing them onto the wound area. However, challenges arise when large areas are affected and donor sites are scarce. In such cases, the available healthy donor skin is often insufficient for autografting, leaving a large portion of the wounded area either ungrafted, allografted, or uncovered, and resulting in poor outcomes. Use of regenerative cells and materials are one possible solution, with cells often demonstrating a better outcome. However, methods to transfer viable cells onto wound areas remain a challenge.
TECHNOLOGY
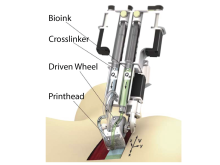
Inventors at the University of Toronto have developed a handheld bioprinter for applying a regenerative treatment to deep skin wounds. The device is principally composed of one chamber containing a bioink (e.g. cells or matrix), a second chamber containing a matrix crosslinker, a microfluidic printhead, and a driven wheel (Fig 1). As the device is guided across the wound, a bioink layer is applied, almost immediately followed by an overlying crosslinker-containing layer. The two layers conform to wound structure whereafter mixing of the layers leads to solidification of the liquids (i.e. gelation) at the wound site. This regenerative gel “bandage” is then responsible for instigating skin formation.
Figure 1. Schematic of the handheld bioprinter for treating skin wounds
COMPETITIVE ADVANTAGE
- High cell viability
- Even layer deposition of treatment
- Large surface area coverage
- Quick application time
- Simple and cost-effective solution
APPLICATIONS
- Treatment of deep skin wounds
INTELLECTUAL PROPERTY STATUS
- PCT Application No. WO2018064778A1 (2016). National phase applications US, EP, JP, AU, CA, CN.
- US Patent Application No. US2020023172A1 (2018). National phase applications: CA, US
PROJECT STATUS
The inventors have built a prototype of the device and tested it in vitro and on wound models in pigs. Using this device, they have shown even layer deposition on curved surfaces and good cell viability (>90%), both of which are important considerations to skin formation. Furthermore, they have demonstrated wound closure after both acellular and cellular treatments (Fig 2), with the cellular treatment displaying a superior healing profile as evidenced by a reduction in inflammation, scarring and contraction. The device has the potential to be used for individual or combined application of many different materials and/or cells.
Figure 2. The skin wound a) before and b) after deposition of the regenerative treatment in pig models. Closure of the wound following c) acellular (matrix) and d) cellular treatment using the handheld bioprinter.