**** Now commercially available (MyoMaxTM) ****
BACKGROUND
Induced pluripotent stem cells (iPSCs), and iPSC-derived cardiomyocytes (iPSC-CMs) offer tremendous promise for basic cardiovascular science, disease modeling, drug efficacy and toxicity testing, personalized medicine, and cell therapy to restore myocardial function during heart failure or post-infarction. However, current iPSC-CMs are physiologically immature in all categories of relevant function, including morphology, contractility, calcium handling, action potential electrophysiology, and metabolism. This is a major limitation towards the application and commercialization of iPSC-CMs.
TECHNOLOGY
Our researchers have developed a novel and defined culture medium for the functional maturation of iPSC-CMs using an iterative, directed-evolutionary algorithm that screened 17 factor additives simultaneously and converged to a high-performing formulation for further characterization.
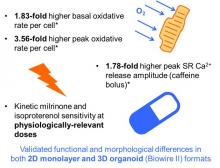
The resulting formulation differs in composition significantly from current gold-standard media (commercial and in literature), and has been validated for compatibility in both pure 2D iPSC-CM monolayers as well as 3D organoid co-cultures with fibroblasts for synergistic maturation using electrical stimulation. iPSC-CMs cultured in this novel formulation have been demonstrated to outperform these validated gold-standard media in quantitative metrics of morphology, contractility, calcium handling, and oxidative metabolism. This maturation medium is currently being further assessed for specific applications in cardiac safety screening and cell therapy.
Furthermore, cells matured in the custom medium have been benchmarked to both commercial and literature gold-standards in multiomics screens (bulk RNA sequencing, global proteomics, and 13C metabolic flux). These results suggest continued advancement of iPSC-CMs cultured in the algorithmically-derived medium toward a more mature model of in vivo function.
COMPETITIVE ADVANTAGE
- Compatible with both 2D iPSC-CM monolayers and 3D co-cultured organoids
- Enhanced cell hypertrophy, elongation, and alignment
- Enhanced Ca2+ handling and sarcoplasmic reticulum function
- Increased per-cell metabolic rate and mitochondrial capacity
- Stratification and enrichment of metabolic, contractile, and SR-associated protein expression by global proteomics
- Not dependent on end-user's experience and technique
APPLICATIONS
- Basic science and in vitro pathophysiology
- In vitro cardiac pharmaceutical screening
- Development of cell therapy
INTELLECTUAL PROPERTY STATUS
- Provisional patent filed (Nov 2021)
PROJECT STATUS
In vitro physiology and omics data collection complete for first manuscript submission (projected for August 2021). Advanced electrophysiology and organoid characterization (using Biowire II platform) is in progress. Cell therapy applications are in planning stages.