BACKGROUND
Antibody binding to antigens results in the formation of immune complexes (ICs), which regulate a variety of functions of the immune system. One pertinent consequence of ICs is the enhanced host immunity against the antigen contained in the complex. Engineered ICs have applications as carriers for vaccines and immunotherapies. However, these applications have been constrained by the limited control over IC structure and composition. Existing methods for synthesizing ICs often yield heterogenous aggregates of uncontrolled size, shape, and antibody valency, resulting in poor pharmacokinetics and clearance. Our approach addresses this by using DNA nanostructures to create ICs with customizable physical properties, along with controlled incorporation of cargoes, providing a programmable system for delivery of immune-modulating drugs and vaccines.
TECHNOLOGY
Inventors at the University of Toronto have used DNA nanostructures to generate synthetic immune complexes that do not exist in Nature. The DNA nanostructures provide customizable 3D scaffolds to display antigens in nanoscale spatial patterns, templating the binding of antibodies during IC formation (Figure 1, left). By tuning the antigen pattern on the DNA nanostructures, researchers are able to program ICs of unique shapes, sizes, and antibody valences, and mediate their delivery to immune cells (Figure 1, right). Furthermore, the technology can incorporate a variety of therapeutic payloads including protein or peptide antigens, adjuvants, and small molecule drugs, making it a versatile platform for vaccine and immune-modulation and applications.
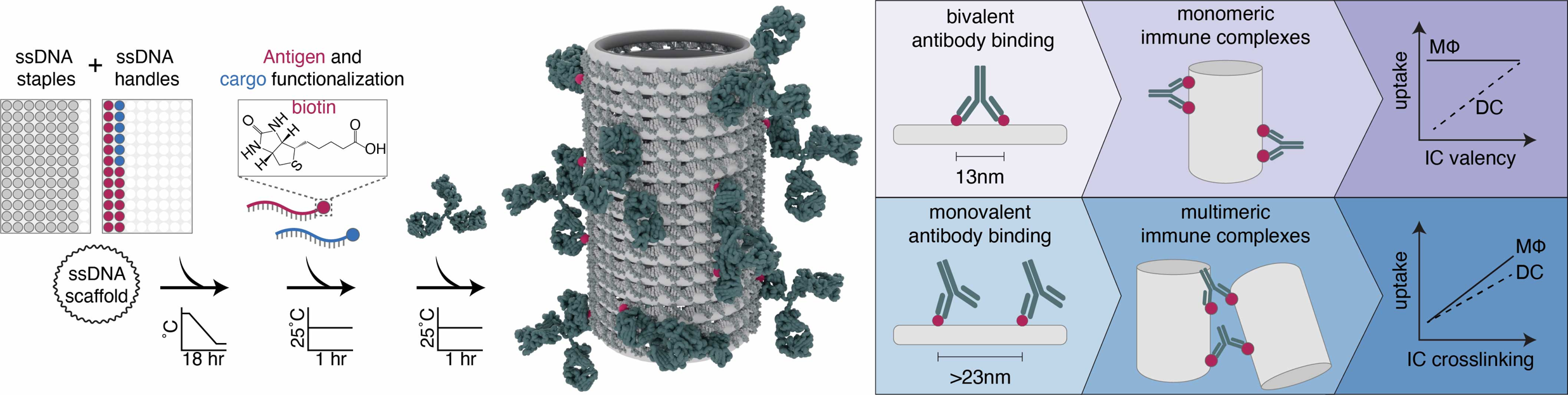
Figure 1. Antigens can be patterned on 3D DNA nanostructure scaffolds to assemble synthetic immune complexes (left). Programmed antigen spacing determines antibody binding to produce highly precise ICs, where antigen valency in either state can be tuned to control either antibody valency (monomeric) or IC size (multimeric). These physical characteristics determine IC uptake in macrophages and dendritic cells (right).
COMPETITIVE ADVANTAGE
- Synthetic immune complexes with customizable size, shape, and composition for tailored, receptor-targeted delivery to immune cells
- First IC platform that can co-deliver one or combination payloads (antigens, peptides, adjuvants, drugs, nucleic acids, etc.) to cells
- Simple, robust, programmable self-assembly that does not require laborious protein engineering and can use both receptor and ligands in their native forms
APPLICATIONS
- Fc receptor-targeted therapeutic delivery – development of IC-based therapeutics for autoimmune disease, infectious disease, and cancer
- Vaccines that leverage Fc-mediated effector functions
- Fundamental immuno-biology discovery
INTELLECTUAL PROPERTY STATUS
- US Provisional (April 2024)
PROJECT STATUS
Design rules for controlling IC structure have been established, and initial study demonstrates promising insights into in vitro cellular interactions. Enhanced receptor-mediated uptake in macrophages and dendritic cells depended on the physical characteristics of the IC (Figure 2). The technology is positioned for translational development, understanding how the physical properties of the IC influence biodistribution in vivo are underway.
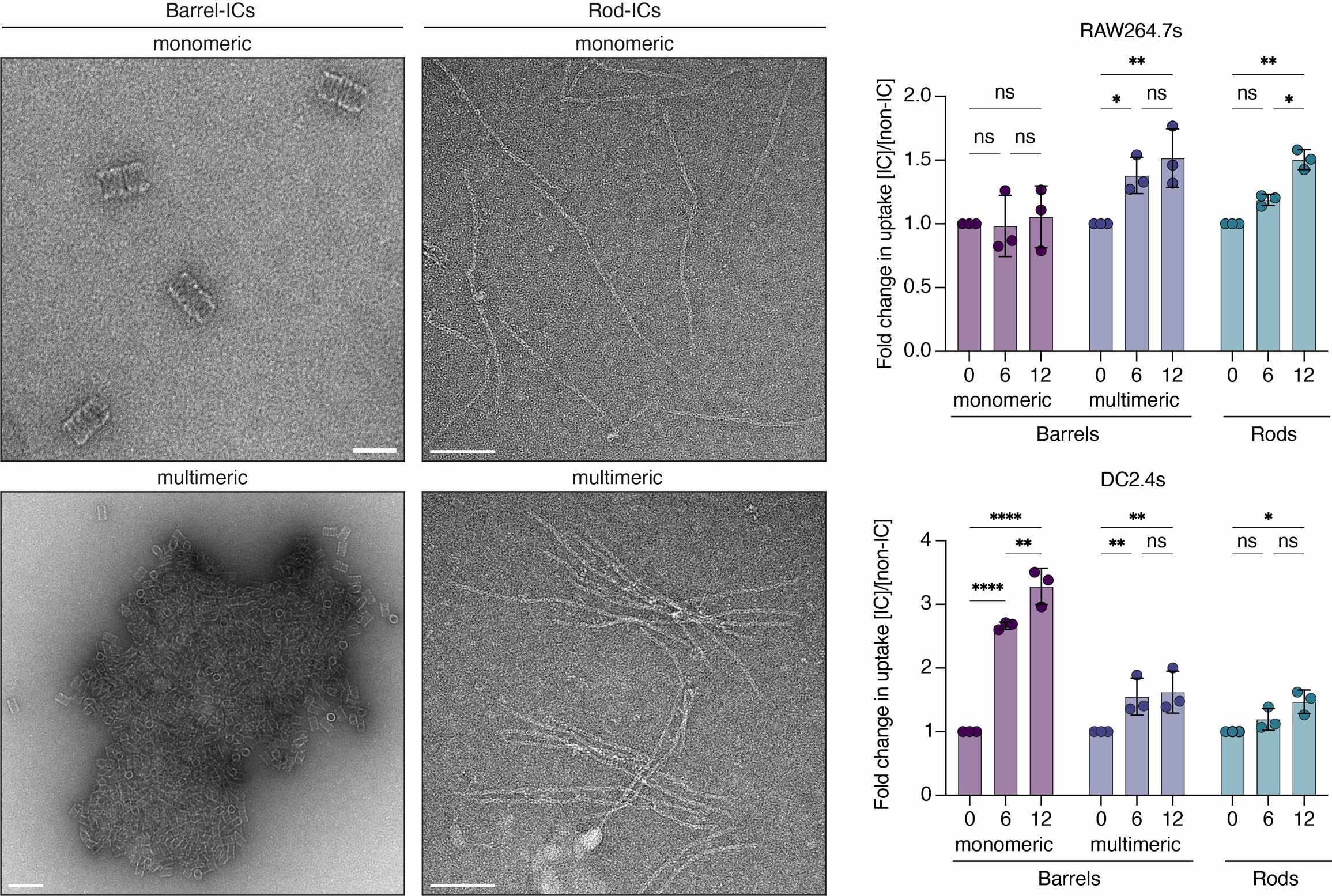
Figure 2. Transmission electron micrographs of monomeric and multimeric ICs, and their respective shapes (left). Fold change in cellular uptake depends on IC shape, size, and antibody valency (right).





