BACKGROUND
Parkinson’s disease is a disabling and progressive neurological disorder characterized by degeneration of dopamine-producing neurons. Current therapies to replace dopamine are only symptomatic and are associated with considerable complications as the disease progresses. No disease-modifying therapies that slow or stop disease progression are available or likely to emerge soon. With Parkinson’s disease affecting over 6 million people worldwide and being the fastest growing neurological disease, this is a huge unmet need in neurodegeneration.
TECHNOLOGY
Researchers at the University of Toronto have developed a platform to identify peptide-based protein-protein interaction inhibitors. They applied this platform to alpha-synuclein based models of Parkinson’s disease and identified several peptide candidates as potential therapeutics. They validated these peptides in a battery of follow-up measurements and optimized their top candidate, which inhibits a previously unknown interaction that they show to be a cause of alpha-synuclein accumulation. Following optimization, the researchers tested this peptide in animal and human models of Parkinson’s disease in which it demonstrated strong efficacy.
COMPETITIVE ADVANTAGE
- Novel screening platform enabled discovery of protein-protein interaction hits
- Lead compound with nanomolar target engagement
- Fully novel target in an under-explored molecular pathway
- Proven in vivo efficacy
APPLICATIONS
- Parkinson’s therapeutic
INTELLECTUAL PROPERTY STATUS
- Provisional patent filed (Dec 2021)
PROJECT STATUS
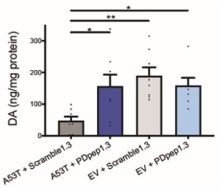
Using our protein-protein interaction screening platform, we identified protein-protein interaction inhibitors upstream of alpha-synuclein that rescue its neurotoxicity. We identified the target of our top hit and optimized it to a compound (PDpep1.3) with nanomolar target engagement. We elucidated that PDpep1.3 has a novel mechanism of action leading to alpha-synuclein degradation, thereby rescuing its toxic effects. We demonstrated that PDpep1.3 reduces alpha-synuclein levels in cultured rat cortical neurons, human fibroblasts harbouring a disease-causing mutation, and iPSC-derived dopaminergic neurons with a disease-causing mutation (Figure 1). Furthermore, PDpep1.3 rescues dopaminergic neurons from alpha-synuclein mediated degeneration in C. elegans and preclinical rat models of Parkinson’s disease (Figure 2).
Figure 1. PDpep1.3 reduces alpha-synuclein protein levels in rat cortical neurons expressing the disease-causing A53T alpha-synuclein mutant (left panel) and normalizes A53T alpha-synuclein levels to those of isogenic controls in dopamine neurons derived from human iPSCs (right panel). A scrambled version of the peptide (Scramble1.3) was used as a control peptide.
Figure 2. In a commonly used rat preclinical model of Parkinson’s disease, viral mediated expression of A53T alpha-synuclein causes degeneration of TH+ dopamine neurons in the substantia nigra compared to empty viral vector (EV). PDpep1.3 reduces degeneration of dopamine neurons in this model (left panel) and rescues dopamine levels in the striatum, which receives projections from the affected substantia nigra (right panel).
TEAM
The team is comprised of leading experts in all relevant fields. Philip M. Kim is an expert on peptide and protein engineering. Lorraine Kalia and Suneil Kalia are clinician-scientists with expertise in molecular mechanisms and preclinical models of Parkinson’s disease. In addition, Lorraine Kalia is a movement disorders neurologist and Suneil Kalia is a stereotactic and functional neurosurgeon, both leaders in the treatment of Parkinson’s disease.