BACKGROUND
HDAC6 plays an important role in neurological, inflammatory, and other diseases; and thus is an important target for therapeutic intervention. To date, there are no FDA-approved HDAC6-targeting drugs and most pipeline candidates suffer from poor target engagement, inadequate brain penetration, and low tolerability. There are five HDAC6 clinical candidates for the treatment of mostly non-CNS cancers as their pharmacokinetic liabilities exclude them from targeting HDAC6-implicated neurological diseases, urging for the development to address these challenges. They also demonstrate off-target toxicity due to limited selectivity, leading to adverse effects in patients. Selective inhibitors have thus been the focus of development over the past decade, though no selective and potent HDAC6 inhibitor has yet been approved to date.
TECHNOLOGY
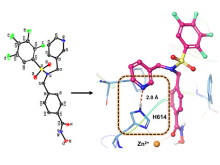
Researchers at the University of Toronto have used a rational, iterative approach to design a 2-site binding mechanism to target HDAC6, which has generated a platform that is the first to overcome core drug-design challenges such as weak binding, off-target toxicities, and poor pharmacokinetic profiles. (Figure 1). HDAX has also designed blood-brain-barrier crossing HDAC6 inhibitors, enabling CNS disease targeting that is not possible with competing molecules. Additional applications possible in Charcot-Marie-Tooth (CMT), neurodegeneration, and heart failure.
Figure 1. Unique patented binding mode is highlighted by X-ray co-crystal structure of a POC compound in the catalytic pocket of HDAC6, translating into improved potency, selectivity, drug half-life and expanding mechanism of action.
COMPETITIVE ADVANTAGE
Over the last 7 years, we have validated our unique platform, predicated on our novel bitopic binding mode (resolved by X-ray crystallography), and differentiated via 3 core features:
- PHARMACODYNAMICS: Strong HDAC6 target engagement: Picomolar HDAC6 potency in contrast to nanomolar HDAC6 potencies in competition discovery pipelines. Significantly improved in cellulo residence time of our HDAC6-based assets upon comparison to leading clinical candidate. We have validated on target activity in vitro, in cellular models and also in vivo.
- SELECTIVITY AND TOLERABILITY: 800-fold selectivity of HDAX candidates compared to 6-fold HDAC6 selectivity of advanced clinical candidates. This has resulted in strong target selectivity in vitro and in vivo, with no concerns of tolerability in our animal studies. Preliminary genotoxicity, cardiac toxicity, and neurotoxicity have also been assessed and show no concerns.
- PHARMACOKINETICS: Commendable in vivo pharmacokinetics profile with added brain penetrance: Demonstrated t1/2 from 2-4h with >50% brain permeability of our drug candidates compared to FDA-approved HDAC inhibitors lacking any brain penetration and known competitor compounds having less than 10% brain penetration, both of which possess t1/2 ~20 min.
APPLICATIONS
- Primary: Chemotherapy-induced peripheral neuropathy (CIPN)
- Secondary: Charcot-Marie-Tooth (CMT), Diabetic Peripheral Neuropathy (DPN), Neurodegeneration, Heart failure
INTELLECTUAL PROPERTY STATUS
- Provisional patent application (Aug 2022)
PROJECT STATUS
Lead optimization stage: Identified a lead compound and currently conducting lead optimization; demonstrated strong PK/PD in vivo, differentiation of target engagement, residence time, selectivity and tolerability from existing compounds, and efficacy in human iPSCs; expecting clinical candidate nomination in 2023.