INTRODUCTION
Psychosis and schizophrenia are associated with dysregulation of multiple neurotransmitter systems, including dopamine, serotonin, gamma-aminobutyric acid, and glutamate. Treatments for these disorders rely on the use of antipsychotics which target several G-protein coupled receptors (GPCRs) such as those for dopamine and serotonin. However, these therapeutics are associated with a range of side effects including extrapyramidal motion, sedation, metabolic syndrome, and weight gain. The ability of the endocannabinoid system, including the cannabinoid CB1 receptor (CB1R), to modulate dopamine neuron signalling offers a route to indirectly correct for the hyperdopaminergic underpinnings of these disorders while possibly avoiding the negative side effects of current antipsychotics. As efforts to target CB1R at the orthosteric site have not yielded beneficial clinical outcomes, allosteric modulation may offer an alternative pathway.
TECHNOLOGY
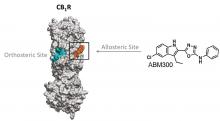
Researchers at the University of Toronto have investigated a novel pharmacological approach to targeting CB1R. They have designed and tested a small molecule (ABM300) that binds to an allosteric site of this receptor (Figure 1) normally occupied by cholesterol. ABM300 partitions into the bilayer, competes with cholesterol, and acts as an inhibitor of CB1R agonist-mediated signalling. It has been tested and characterized both in vitro and in vivo. where it was shown to have comparable to better effects than the approved schizophrenia and bipolar disorder drug, olanzapine.
Figure 1. Structure of CB1R and positions of the othosteric (cyan) and allosteric (orange) sites. ABM300 binds to the allosteric position.
COMPETITIVE ADVANTAGE
- Novel pharmacological approach (allosteric inhibition) to targeting CB1R
- Dysregulated behaviors reduced in two mouse models of hyperdopaminergia
- GluN1KD mouse model - Reduced hyperactivity (-50%), stereotypy (-25%) and vertical activity (-60%)
- DATKO mouse model – Reduced hyperactivity (-40%), stereotypy (-30%), vertical activity (-60%); improved response to pre-pulse inhibition stimuli
- Comparable to better phenotypic improvements elicited than olanzapine (current treatment for schizophrenia and bipolar disorder)
- Rescue of phenotypes occurred without adverse anxiogenic-like or cannabimimetic effects traditionally observed with orthosteric CB1R inverse agonists
APPLICATIONS
- Treatment of psychosis and schizophrenia
INTELLECTUAL PROPERTY STATUS
- National Phase: US, CA
PROJECT STATUS
- ABM300 was characterized in vitro (receptor binding, β-arrestin2 recruitment, ERK1/2 phosphorylation, cAMP inhibition) in hCB1R CHO cells and hCB1R HEK cells. It was also tested in vivo (anxiety-like behaviors, cannabimimetic effects, novel environment exploratory behavior, pre-pulse inhibition, conditioned avoidance response) to assess the effects of the compound in dysregulated behaviors within two transgenic models (GluN1-Knockdown (GluN1KD) and Dopamine Transporter Knockout (DATKO)) of hyperdopaminergia. Results from one of the models (GluN1KD) was benchmarked to olanzapine, a treatment for schizophrenia and bipolar disorder.