BACKGROUND
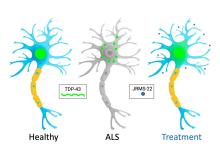
TAR DNA-binding protein 43 (TDP-43) plays a crucial role in the pathogenesis of several neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS), frontotemporal lobar degeneration (FTLD), and Alzheimer's disease (AD). Under normal conditions, TDP-43 is involved in RNA metabolism, including splicing regulation, mRNA stability, and miRNA biogenesis. However, in neurodegenerative diseases, TDP-43 often becomes mislocalized to the cytoplasm, where it forms insoluble aggregates and inclusions in neurons and glial cells. These aggregates disrupt normal cellular functions, contributing to neurodegeneration. Recent research has focused on modulating TDP-43 activity and preventing its aggregation as potential therapeutic strategies. However, current therapeutic approaches face deficiencies, such as the inability to effectively target and reverse TDP-43 aggregation and mislocalization. Addressing these challenges is essential for advancing treatment options for ALS and other TDP-43-related neurodegenerative diseases.
TECHNOLOGY
Researchers at the University of Toronto have discovered a small molecule ‘JRMS’ that harnesses the non-canonical chaperone activity of importin-β1 (Kpnβ1) to prevent and reverse protein aggregation as a therapeutic for ALS, and the broader neurodegenerative diseases spectrum. ‘JRMS’ increases Kpnβ1cytoplasmic localization, increasing its interaction with aggregation-prone proteins like TDP-43, mutant SOD1 and alpha-synuclein. ‘JRMS’ prevents and reverses TDP-43 toxic aggregates leading to an increase TDP-43 normal nuclear localization in cells, human iPSCs-neurons, mouse primary cortical neurons, organotypic slices and a mouse model.
COMPETITIVE ADVANTAGE
- Novel mechanism harnessing endogenous cellular machinery
- Reducing TDP-43 aggregation resulting in refolding and restoration of nuclear localization
- Targeting the pathology at its root cause for a disease-modifying treatment
- Small molecule with positive pharmacokinetics
APPLICATIONS
- TDP-43 pathologies
- ALS, FTD, Alzheimer’s Disease
- ALS, FTD, Alzheimer’s Disease
INTELLECTUAL PROPERTY STATUS
- PCT Application (Oct 2024)
PROJECT STATUS
This project is at the early lead optimization stage. The mechanism has been elucidated and a molecule for lead optimization identified. Medicinal chemistry is being conducted in collaboration with LifeArc, a partner organization investing significant milestone-dependent developmental resources with a view to licensing a developmental candidate into Neuropeutics in ~3 years.