BACKGROUND
Monoclonal antibodies are the primary treatment option for targeted cancer therapies, yet they pose significant limitations within the clinic. Current antibodies target surface markers that are present on both cancer and healthy cells triggering off-target effects following administration. Consequently, almost all monoclonal antibodies cause adverse reactions ranging from minor to severe (grade 3 or higher) responses and at times, treatment-induced deaths. By contrast, cancer cells possess unique markers in the form of mutated proteins that are located intracellularly and are absent within healthy cells. The fragments of these proteins are present on the cancer cells’ surface as peptides that are displayed on their major histocompatibility complex 1 (MHCI) molecules. For antibody therapies the capacity to target these peptide MHCI molecules would significantly mitigate off-target effects, resulting in a safer therapy.
TECHNOLOGY OVERVIEW
Our technology is a vaccine that induces patients’ own B cells to produce antibodies that target cancer exclusive peptide MHCI molecules. Specifically, we have developed an antigen design that efficiently delivers a single-chain cancer peptide-MHCI (pMHCI) molecule to the lymph node (Figure 1). This antigen design uniquely triggers the production of peptide MHCI targeting antibodies from B cells while traditional antigens (i.e. peptides or whole protein from which the peptides are derived from) fail to do so. The antigens can be delivered through multiple vaccine modalities such as recombinant proteins or encoded in mRNA. Overall, we provide a vaccine platform that induces continuous production of cancer exclusive antibodies directly within patients.
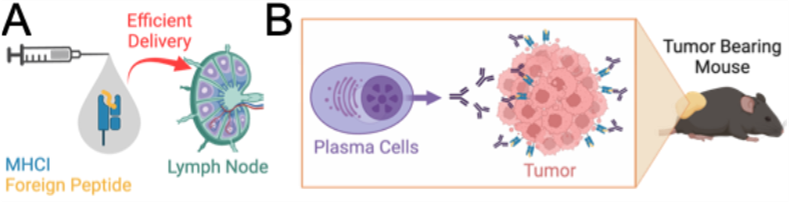
Figure 1. Overview. (A) B cell antigen that uses MHCI molecule displaying cancer peptides. (B) Secretion of Abs by plasma cells that exclusively target cancer cells.
COMPETITIVE ADVANTAGE
Our vaccine is a disruptive platform that, for the first time, mobilizes our B cells towards effective cancer therapy. By placing the onus onto B cells to discover and mass produce antibodies, our vaccine overcomes several limitations associated with the monoclonal antibody R&D and production. It offers personalization potential; its modular design allows incorporation of multiple patient-specific neoantigens.
APPLICATIONS
Our vaccine is intended to be used for cancer therapies as a form of targeted treatment option.
INTELLECTUAL PROPERTY STATUS
-
US Provisional Filed (May 2024)
PROJECT STATUS
Proof-of-concept studies have been conducted. We have developed and characterized our antigen through in vitro assays. We also verified that this antigen can induce specialized antibodies that recognize peptide MHCI molecules within a mouse model. Moreover, vaccination with our antigen protects against a melanoma challenge in prophylactic (Figure 2) and active therapy settings. Currently, we are conducting more in-depth analyses of the antibody response as well as evaluating our vaccine’s efficacy in treating existing tumors.
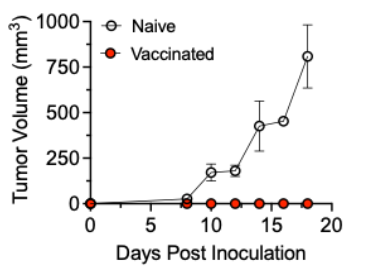
Figure 2. Prophylactic efficacy of our vaccine. Naive or vaccinated immune competent mice were subcutaneously inoculated with murine melanoma cells. Subsequent tumor growth was monitored.
KEYWORDS
TCR-like antibodies, B cell vaccine, peptide-MHCI, psMHCI, cancer immunotherapy, mRNA vaccine, neoantigen, nanoparticle vaccine, targeted delivery