BACKGROUND
Age-related macular degeneration (AMD) is a leading cause of vision loss in older adults, with dry AMD accounting for the majority of cases. In its advanced form, known as geographic atrophy (GA), dry AMD leads to irreversible retinal cell death and central vision loss. Until recently, treatment options were limited, but two biologics—pegcetacoplan (Syfovre) and avacincaptad pegol (Izervay)—received FDA approval in 2023 as the first therapies specifically targeting GA. Both are administered via monthly or bimonthly intravitreal injections, and work by modulating the immune system to slow retinal degeneration. Clinical trials showed they can reduce the progression of GA by approximately 14–20%, but neither drug restores lost vision. Treatment gaps remain significant: the burden of frequent eye injections, modest efficacy, approval only for advanced disease, and risks such as inflammation, bleeding, and a temporary increase in eye fluid pressure pose challenges for to use. These limitations underscore the need for more effective, less invasive, and safer therapies for dry AMD.
TECHNOLOGY
Researchers at the University of Toronto have developed inhibitors for Redox effector factor 1 (Ref-1) for treatment of dry AMD. Ref-1, a redox-mediated transcriptional regulator, has previously been implicated in inflammation and angiogenesis in the eye and elsewhere but not in retinal degeneration. They have demonstrated that systemic administration of APX2009, a safe and selective Ref-1 inhibitor, significantly mitigates retinal degeneration in a sodium iodate-induced murine model with features of dry AMD (Figure 1). They have further demonstrated preservation of barrier function and morphology in human iPSC-derived retinal pigment epithelium (RPE) cells exposed to NaIO₃. These findings establish Ref-1 inhibition as a promising systemic therapy for dry AMD and related retinal degenerative diseases.
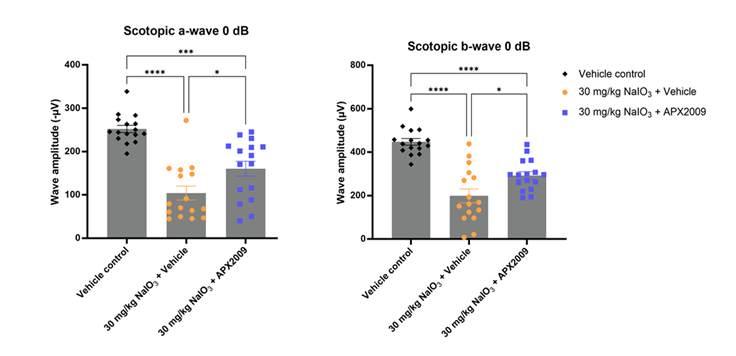
Figure 1. APX2009 reduces retinal degeneration in mice after NaIO3-induced damage. Representative scotopic a-wave and b-wave amplitudes from electroretinograms (ERGs) of control (black), diseased (orange) and treated (blue) mice. Photopic benefits were less pronounced.
COMPETITIVE ADVANTAGE
- Novel Mechanism: Targets the redox function of Ref-1, a pathway not addressed by current AMD therapies.
- Systemic Delivery: Unlike current intravitreal therapies, APX2009 is safe and effective via systemic administration, offering a less invasive treatment option.
- Demonstrated Efficacy:
- Significantly reduces retinal thinning in vivo; maintains RPE TEER and cell morphology in vitro.
- Preserves both RPE and photoreceptor integrity and function, as shown by ERG and histological analysis.
- Broad Therapeutic Potential: May be applicable to other oxidative stress-related retinal diseases, including Sorsby fundus dystrophy.
APPLICATIONS
- Treatment of dry age-related macular degeneration (AMD)
INTELLECTUAL PROPERTY STATUS
- PCT Application (May 2025)
PROJECT STATUS
Proof-of-concept demonstrated in vivo using a validated murine model and in vitro using human iPSC-derived RPE cells. Mice treated with APX2009 preserved retinal structure and function, as evidenced by improved fundus appearance, OCT imaging, ERG waveforms, and immunohistochemical markers (RPE65 and rhodopsin). In vitro, APX2009 also preserved barrier function and morphology in human iPSC-derived RPE cells exposed to NaIO₃.
KEYWORDS
dry AMD, geographic atrophy, retinal degeneration, Ref-1 inhibitor, oxidative stress, retinal pigment epithelium (RPE) protection, systemic therapy